Abstract
Background:
Diffuse large B-cell lymphoma (DLBCL) is an aggressive lymphoid malignancy with heterogeneous clinical outcomes following treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). [18F] fluorodeoxyglucose (FDG) - positron emission tomography (PET)/computed tomography (CT) imaging is routinely used in staging and response assessment for DLBCL at diagnosis and after treatment. However, the prognostic value of PET during treatment (interim) and of PET-derived metrics measuring tumor burden and metabolism remains unresolved. Such PET-derived metrics include tumor maximum standardized uptake value (SUV); metabolically active tumor volume (MTV), calculated as the total volume of tumor with FDG uptake; and total lesion glycolysis (TLG), the sum of tumor volume weighted by the intensity of FDG uptake. We investigated the predictive value of pre-treatment, interim and end-of-treatment (EOT) MTV and TLG on progression-free survival at 24 months (PFS24) in patients with DLBCL treated with R-CHOP.
Methods:
Patients with DLBCL treated at Emory University between 2005-2016 were eligible. Cases were included if there was a diagnosis of DLBCL confirmed by record review, available information on date of diagnosis, date of last contact or date of death. Analyses were restricted to patients who received R-CHOP and had PET/CT scans available before, during, and after treatment. Maximum SUV, MTV, and TLG were calculated using MIM software for tumor with an SUV threshold > 4. Univariate analysis was used to examine the relationship between interim MTV and post-treatment MTV, DLBCL subtype (classified as either germinal center B-cell (GCB) or non-GCB by the Hans algorithm), and LDH (defined as high > 200 or low < 200) at time of diagnosis. Logistic regression was used to examine the predictive value of pre-treatment and interim MTV on EOT PET results as well as the relationship between LDH and pretreatment MTV. Cox proportional hazard models were used to estimate PFS24 hazard ratios (HR) and 95% confidence intervals (CI) for positive pre-treatment MTV, interim MTV, EOT MTV, subtype and LDH at diagnosis.
Results:
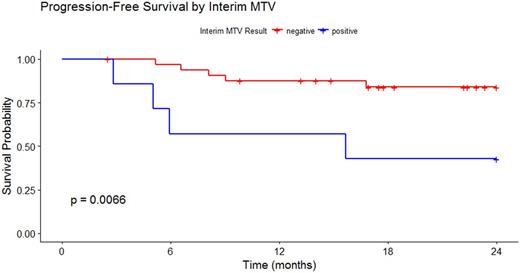
Pre-treatment PET/CT scans for 55 patients were identified. The mean pre-treatment MTV was 304 ml (range 4.26 - 1,488 ml) and mean TLG was 3068 ml*SUVbw (range 24.48 - 16,180 ml*SUVbw). The mean pre-treatment MTV was 261 ml (range 4.26 - 1,327 ml) and mean TLG was 2033.768 ml*SUVbw (range 24.48-8,783 ml*SUVbw) for GCB DLBCL and were 383 ml (range 12.82 - 1,488 ml) and 4344.058 ml*SUVbw (range 85.29 - 16,180 ml*SUVbw) for non-GCB DLBCL, respectively. MTV and TLG were undetectable in 79% of interim scans and 74% of post-treatment scans. Receiver operating characteristic curve analysis determined optimal cutoffs of pre-treatment MTV at 121 ml, interim MTV at 0 ml and EOT MTV at 4.68 ml for predicting PFS. Elevated LDH was strongly associated with a high pre-treatment MTV (p = 0.0006). Controlling for pre-treatment MTV, a positive interim MTV was highly correlated with (0.86) and a significant predictor of a positive EOT MTV (p = 0.03). Interim MTV > 0 ( HR 5.51, CI 1.13, 26.79) and EOT MTV > 4.68 (HR 10.75, CI 1.31, 105.48) were significant predictors of PFS24, controlling for pre-treatment MTV.
Conclusions:
PET-derived metrics of pre-treatment and interim MTV offer significant predictive value for end of treatment response and progression-free survival, and can guide future response-adapted treatment approaches for DLBCL patients that build on the R-CHOP backbone. Interim and EOT MTV provide value in DLBCL risk stratification and can be incorporated into new integrative prognostic models. Larger outcomes studies based on secondary analyses of clinical trials data or large observational cohort studies are needed to confirm these findings.
Flowers: Clinical Care Options: Research Funding; V Foundation: Research Funding; Burroughs Welcome Fund: Research Funding; Janssen Pharmaceutical: Research Funding; Educational Concepts: Research Funding; Acerta: Research Funding; Gilead: Consultancy; Seattle Genetics: Consultancy; Research to Practice: Research Funding; Prime Oncology: Research Funding; Bayer: Consultancy; Pharmacyclics LLC, an AbbVie Company: Research Funding; National Cancer Institute: Research Funding; National Institutes Of Health: Research Funding; OptumRx: Consultancy; Millennium/Takeda: Research Funding; Abbvie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Onyx: Research Funding; Infinity: Research Funding; Spectrum: Consultancy; Genentech/Roche: Consultancy, Research Funding; TG Therapeutics: Research Funding; Eastern Cooperative Oncology Group: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal